Pressroom
Gestalt in the Press &
Out in the Industry
Press Releases

Spokane, WA, October 6, 2025 — Gestalt is proud to announce the release of PathFlow Version 6, the latest evolution of its award-winning digital pathology platform. This major update introduces a dynamic, high-powered field of view designed to elevate the diagnostic experience for pathologists, along with a dedicated tumor board workflow module that streamlines multidisciplinary collaboration, in addition to other features. PathFlow Version 6 delivers a robust visual interface that enables pathologists to navigate their images with greater precision, speed, and clarity—supporting complex case reviews and high-volume workflows without compromise. The enhanced field of view is optimized for performance across diverse specimen types, and in a manner that is familiar and comfortable to them. By providing pathologists with this embedded high-power field of view, they are able to standardize the quantitative assessment of specific cells or features within a tissue sample, which is essential for cancer diagnosis, tumor grading, and determining patient prognosis, ensuring diagnostic confidence every time. The newly developed tumor board module provides the focused workflow needs for supporting real-time collaboration across oncology teams, radiologists, and pathologists. With configurable workflows, secure data sharing, and the ability for both admins and pathologists to easily add, update and remove cases and images institutions can now conduct tumor board reviews with greater efficiency and clinical alignment. “ This release reflects our commitment to empowering pathologists with tools that not only enhance diagnostic accuracy but also foster meaningful collaboration, ” said Lisa-Jean Clifford, President of Gestalt Diagnostics. “ PathFlow Version 6 is built for the demands of modern pathology and the future of precision medicine. ” PathFlow V.6 is available immediately to existing customers and new partners seeking scalable, interoperable digital pathology solutions. It is being featured during DPA’s Pathology Visions Conference in San Diego this week. Come to booth #600 for a personalized demonstration. Not going to Visions? Reach out for more information. -- About Gestalt Gestalt transforms pathology through an intelligent, configurable, vendor-neutral, and AI-driven digital workflow that provides true interoperability enabling pathologists to diagnose diseases faster and more efficiently. Our PathFlow solution consists of professional, education, and research modules for ease of mixing and matching the digital needs of your facility in a single solution, freeing pathologists from tedious, repetitive, and manual tasks, allowing them to focus on their expertise – providing invaluable experience where it matters most. To learn more, visit www.gestaltdiagnostics.com and follow @Gestalt on LinkedIn

Spokane, WA – October 2, 2025 — Gestalt, a leader in digital pathology and AI-driven laboratory solutions, is proud to announce that Tulsa Medical Lab (TML) has selected PathFlow®as their digital pathology solution, marking a major step forward in the lab’s strategy to streamline operations, enhance diagnostic accuracy, and accelerate the adoption of AI-driven workflows. With this partnership, TML aims to unify its diagnostic process across multiple laboratory information systems, enabling pathologists to access all of their cases through a single login and in one consolidated worklist. The implementation of PathFlow will also give TML access to integrated AI tools designed to support efficiency and accuracy throughout the diagnostic process. “ Gestalt is one of the best vendors I've ever worked with in my 20+ years as a pathologist, ” said Dr. Anne V. Herdman Royal, Managing Partner, TML. “ They are honest, clear in delivering on their goals and promises, and they are genuinely interested in improving the ease and efficiency of the pathologist's digital pathology experience. ” Tulsa Medical Lab selected Gestalt for its pathologist-focused approach, robust platform features, and ability to support scalable innovation. The lab’s long-term goal is to create a “one-stop shop” digital-based system that streamlines access to all pathology cases, empowering its team to work more collaboratively and efficiently while laying the foundation for future AI-enabled diagnostic workflows. “ We are proud to partner with Tulsa Medical Lab as they take this important step toward digital transformation ,” said Lisa-Jean Clifford, President of Gestalt Diagnostics. “ Their vision for a unified, pathologist-centered digital environment aligns closely with our mission to deliver scalable, interoperable solutions that improve diagnostic efficiency and patient care. ” Through this collaboration, Gestalt continues its commitment to transforming laboratory workflows by providing innovative technology, strategic partnerships, and purpose-driven solutions that advance healthcare outcomes worldwide. About Gestalt Gestalt transforms pathology through an intelligent, configurable, vendor-neutral, and AI-driven digital workflow that provides true interoperability enabling pathologists to diagnose diseases faster and more efficiently. Our PathFlowTM solution consists of professional, education, and research modules for ease of mixing and matching the digital needs of your facility in a single solution, freeing pathologists from tedious, repetitive, and manual tasks, allowing them to focus on their expertise – providing invaluable experience where it matters most. To learn more, visit www.gestaltdiagnostics.com and follow @Gestalt on LinkedIn About Tulsa Medical Laboratory Tulsa Medical Laboratory is a CAP-accredited, independently owned anatomic pathology group serving physicians, clinics, and hospitals throughout Northeastern Oklahoma. The laboratory provides a comprehensive range of diagnostic services, including histopathologic evaluations, cytology interpretations, bone marrow examinations, clinical consultations, blood bank services, peripheral blood and other body fluid smear consultations, and frozen section evaluations. Dedicated to delivering accurate, timely, and patient-centered care, Tulsa Medical Laboratory combines deep expertise with a commitment to quality, innovation, and collaboration. All of which are advancing the practice of pathology and improving healthcare outcomes for the communities it serves.

Spokane, WA – October 1, 2025 — Gestalt, a leader in digital pathology and AI-driven laboratory solutions, is proud to announce that OmniPathology, a high-complexity independent pathology laboratory has selected their digital solution, PathFlow. This partnership marks a significant step forward in transforming diagnostic workflows through leading-edge technology, operational excellence, and a shared vision for global impact. By integrating Gestalt’s award-winning digital pathology platform with OmniPathology’s advanced molecular and anatomic pathology services, the two organizations are setting a new standard for precision, scalability, and interoperability in laboratory diagnostics. The collaboration will streamline case management, accelerate turnaround times, and enhance diagnostic accuracy—delivering measurable improvements for clinicians and patients alike. “ At Gestalt, we believe innovation must be paired with purpose ,” said Lisa-Jean Clifford, President of Gestalt. “ Our partnership with OmniPathology is not only about elevating laboratory performance—it’s about laying the groundwork for diagnostic access in underserved communities around the world. ” OmniPathology’s commitment to excellence and patient-centric care aligns seamlessly with Gestalt’s mission to modernize pathology through open, scalable platforms. Together, the organizations will explore initiatives aimed at extending digital diagnostic capabilities to regions with limited healthcare infrastructure, leveraging AI and cloud-based technologies as learning and teaching tools to bridge gaps in care. “ At OmniPathology, our commitment to innovation goes beyond adopting new tools. It’s about shaping the future of diagnostics. Partnering with Gestalt allows us to combine their world-class AI capabilities with our best-in-class leadership in GI pathology, creating powerful synergies that will enhance precision, efficiency, and improve outcomes for patients, ” said Dr. Mohammad Kamal, Founder and CEO of OmniPathology. “ Together, we see tremendous opportunities to advance pathology, accelerate discoveries in clinical research, and make a meaningful impact on global health. ” The partnership underscores both organizations’ dedication to innovation, education access, and continuous improvement—setting the stage for broader industry adoption and meaningful global health outcomes. About Gestalt Gestalt transforms pathology through an intelligent, configurable, vendor-neutral, and AI-driven digital workflow that provides true interoperability enabling pathologists to diagnose diseases faster and more efficiently. Our PathFlow solution consists of professional, education, and research modules for ease of mixing and matching the digital needs of your facility in a single solution, freeing pathologists from tedious, repetitive, and manual tasks, allowing them to focus on their expertise – providing invaluable experience where it matters most. To learn more, visit www.gestaltdiagnostics.com and follow @Gestalt on LinkedIn About OmniPathology OmniPathology is a leading gastrointestinal pathology and advanced molecular diagnostics laboratory based in Pasedena, Calif. The company delivers academic-level, state-of-the-art pathology services and cutting-edge molecular testing with expertise in infectious disease, cancer diagnostics, and proprietary assay development. Committed to innovation, quality, and precision, OmniPathology partners with clinicians worldwide to improve patient outcomes through accurate, timely, and advanced diagnostic solutions. Learn more at www.omnipathology.com
Publications

Featured in The Pathologist , November 2025 We’re proud to share that Lisa-Jean Clifford, President of Gestalt Diagnostics and President of the Association for Pathology Informatics (API), has been recognized as one of the Leading Voices on The Pathologist Power List 2025 — a curated roster of 50 influential leaders shaping the future of pathology and laboratory medicine. The Power List 2025 celebrates professionals whose ideas, innovation, and leadership are moving the profession forward in meaningful ways. This year’s edition emphasizes voices advancing patient care, inspiring change, and tackling the complex challenges facing pathology today. In her Power List feature, Lisa-Jean Clifford addressed the question: What needs to change in order to maximize pathology’s impact on patient care, and how has your work advanced patient impact? Her core message highlights the need for modern, interoperable, and fully integrated digital pathology systems. According to Clifford, overcoming fragmentation in laboratory information systems and outdated workflows is essential to enable pathologists to deliver faster, more accurate, and more actionable diagnoses — a prerequisite for pathology to fully influence clinical decision-making. Drawing on her years of leadership in healthcare technology and pathology informatics, she shared how her work has advanced patient care: Spearheading development of integrated digital platforms that unify lab data across clinical, anatomic, microbiology, and molecular domains. Driving interoperability and EMR integration to accelerate diagnostic workflows and reduce errors. Advancing digital pathology and AI-enabled solutions — such as enterprise viewers that integrate LIS, EMR, and AI tools — to improve diagnostic speed, accuracy, and risk reduction. Mentoring early-career pathologists and speaking widely on the role of informatics and digital transformation in elevating patient impact. Clifford’s inclusion reflects her longstanding commitment to innovation in pathology, both through technology and professional engagement. The Power List affirms her influence alongside peers driving pathology into the next chapter of clinical relevance and patient-centered care. Read Lisa-Jean Clifford’s full Power List contribution and explore all honorees on The Pathologist.

Featured in Medical Laboratory Observer - MLO, September 2025 Written by Lisa-Jean Clifford, President, Gestalt Fast, accurate diagnosis of men’s most pressing health issues can dramatically improve outcomes, reduce treatment complexity, and enhance overall quality of life. By identifying conditions early—from heart disease to cancers—clinicians can initiate timely interventions that save lives, preserve function, and lower long-term healthcare costs. Of these conditions, all but diabetes and unintentional injury are most often being diagnosed, and treatment plans being determined, based upon information coming from the laboratory. Although, diabetes can lead to health problems that would also land in the direct purview of the laboratory as well. So, let’s dive into each of these a bit. Lung cancer Men are more likely to be diagnosed with lung cancer and less likely to survive it compared to women. The rate of new cases in 2019 was 23% higher in men than women (59 versus 48 per 100,000, respectively), and the five-year survival rate was 39% higher among women than men (30% versus 21%, respectively).1 Skin cancer Skin cancer is the most common of all human cancers. About 1 in 5 Americans will get some type of skin cancer in their lifetime, and more than two people die of skin cancer every hour in the United States. Skin cancer affects people of all skin colors. There are three major types of skin cancers: basal cell carcinoma, squamous cell carcinoma (SCC), and melanoma. The first two skin cancers are grouped together as nonmelanoma skin cancers.2 Colorectal cancer By 2030, cases of colorectal cancer in people under 50 are expected to double.3 The death rate from colorectal cancer for people ages 20 to 54 rose between 2004 and 2014. Before then the death rate had been going down (American Cancer Society). Most people under 50 with colorectal cancer do not have a family history of the disease (American Cancer Society). Black people of all ages, including young adults, are more likely to get and to die from it than any other group. Colon and rectal cancers are both cancers of the intestine. However, their treatments are quite different. Colon cancer Colon cancer is the third most common type of cancer in the United States. However, it is also one of the most curable, and survival rates have been increasing over the past several years. Awareness and early detection are key—colon cancer can often be caught in its early stages, when treatment can be most effective. There are several different ways to test for colon cancer. After reviewing clinical history and doing a basic medical exam, a physician will most likely perform a colonoscopy. Treatment often involves surgery, chemotherapy, and/or radiation. Rectal cancer The rectum sits in a tight space in the body, narrowly separated from other organs and structures in the pelvic cavity. As a result, complete surgical removal of rectal cancer is challenging and complex There are several different ways to test rectal cancer. The most common path for diagnosis will include an ultrasound to see how far through the rectal wall a cancer has grown and a colonoscopy. Rectal cancer often requires more than one type of treatment and much like colon cancer usually involves surgery and a combination of chemotherapy and/or radiation treatments. However, given the complex nature of the rectum, it also often involves partial or total reconstructive surgery or will result in a colostomy bag. Testicular cancer The American Cancer Society’s estimates for testicular cancer in the United States for 2025 are: About 9,720 new cases of testicular cancer diagnosed About 600 deaths from testicular cancer The incidence rate of testicular cancer has been increasing in the United States and many other countries for several decades. The increase is mostly in seminomas. Testicular cancer is not common: about 1 of every 250 males will develop testicular cancer at some point during their lifetime. The average age of males when first diagnosed with testicular cancer is about 33. This is largely a disease of young to middle-aged men, but approximately 6% of cases occur in children and teens and approximately 8% occur in men over 55. Because testicular cancer usually can be treated successfully, the mortality rate for this type of cancer is very low: about 1 in 5,000. Artificial intelligence and machine learning: AI algorithms now analyze ECGs, imaging scans, and clinical data to flag subtle abnormalities faster than traditional methods. Predictive models can forecast disease progression and personalize follow-up intervals. Prostate cancer Prostate cancer ranks among the top cancers in men, especially those over 50. It often grows slowly, allowing many men to remain asymptomatic for years. Early and precise diagnosis of prostate cancer relies on identifying molecular signals—biomarkers—that distinguish healthy tissue from malignancy. Biomarkers guide decisions about who needs a biopsy, what treatments to pursue, and how to monitor treatment response and disease progression. Advances in molecular testing and imaging now allow clinicians to tailor care based on each man’s unique cancer profile. The role of biomarkers in prostate cancer: A biomarker is any measurable molecule in the body that reflects normal or disease processes. In prostate cancer, biomarkers can indicate tumor presence, aggressiveness, and likelihood of recurrence. Incorporating these markers into routine evaluation helps avoid unnecessary biopsies, reduces overtreatment, and pinpoints the most effective therapies for each patient. Mesothelioma Men are significantly more affected by mesothelioma, a rare type of cancer that develops in a layer of tissue that covers most internal organs. Men are over four times more likely to contract mesothelioma, because 80 percent of people with this cancer report at least one exposure to asbestos. Men over 65 who have worked in trade occupations or the military are at the highest risk for this kind of cancer. Idiopathic pulmonary fibrosis Idiopathic pulmonary fibrosis (IPF) is another type of cancer found predominantly in men — 70% of all cases. It is a disease that causes scarring of the lungs. Idiopathic means it has no known cause. IPF symptoms usually appear later in life, with most people being diagnosed over age 50. Symptoms most often include shortness of breath, a dry cough, weight loss, and fast, shallow breathing. IPF does not have a cure and is progressive but can be slowed with medication and treatment, which may also alleviate symptoms for the patient. Viruses In the laboratory, viral infections can be confirmed by multiple methods. Diagnostic virology has changed a great deal due to the increase in molecular techniques and increased clinical sensitivity of serological assays. A large variety of samples can be used for virological testing. The type of sample sent to the laboratory often depends on the type of viral infection being diagnosed and the test required. Cardiovascular disease (CVD) The diagnosis of CVD often begins with identifying clinical symptoms that may indicate underlying heart problems. While some individuals may experience obvious warning signs such as chest pain, shortness of breath, or palpitations, others may have no or atypical symptoms that impede early detection. Fatigue, dizziness, swelling in the legs, and unexplained weakness can also be indicative of cardiovascular issues. In some cases, symptoms appear only during physical exertion, while in others, they occur unpredictably at rest. Beyond traditional diagnostic tests, advanced imaging techniques have revolutionized the early detection of cardiovascular disease. Coronary artery calcium scoring performed using computed tomography (CT) helps assess the presence and extent of calcified plaques in the coronary arteries. This test is useful for identifying those who are not yet displaying symptoms of heart disease. Magnetic resonance imaging (MRI) offers high-resolution images of the heart’s structure and function, making it a valuable tool for diagnosing congenital heart disease, myocarditis, and fibrosis. Similarly, cardiac computed tomography angiography (CCTA) provides detailed images of coronary arteries, allowing clinicians to detect narrowing or blockages with high precision. Laboratory tests and biomarkers for CVD diagnosis: Blood tests play a key role in diagnosing and monitoring the progression of cardiovascular disease. High levels of cholesterol, triglycerides, and inflammatory markers such as C-reactive protein indicate an increased risk of atherosclerosis. Similarly, tests measuring levels of troponin are essential for diagnosing acute myocardial infarction. Other biomarkers, such as B-type natriuretic peptide (BNP), help assess heart failure severity, while glucose and hemoglobin A1c levels provide insights into diabetes-related cardiovascular risks. Latest advancements in the diagnosis of CVD: The diagnosis of CVD has evolved significantly with the integration of leading-edge technologies such as artificial intelligence, machine learning, and advanced imaging techniques. AI-powered algorithms now assist in analyzing ECGs and imaging scans, allowing for faster and more accurate diagnosis. Wearable devices with real-time monitoring identify irregular heart rhythms and provide early indication of heart disease. Genetic testing enables physicians to assess hereditary risks and predict cardiovascular conditions before symptoms are present. All of these advancements improve how CVD is diagnosed, leading to more personalized and proactive healthcare. Genetic testing helps in the diagnosis of CVD: Genetic testing plays a key role in identifying individuals at risk of developing cardiovascular disease. By analyzing specific gene mutations associated with inherited heart conditions, such as hypertrophic cardiomyopathy and familial hypercholesterolemia, physicians can detect heart disease before symptoms appear. Genetic profiling can also help determine an individual’s response to medications, allowing for more personalized treatment plans. Artificial intelligence improves the accuracy of diagnosis: Artificial intelligence is transforming how cardiovascular disease is diagnosed by enhancing the interpretation of medical imaging, ECGs, and patient data. AI-driven algorithms analyze large volumes of clinical data to identify patterns that can be missed by humans, leading to earlier and more accurate diagnoses. Machine learning models can also predict the likelihood of heart disease progression, enabling proactive treatment plans. AI applications in radiology help improve the detection of arterial blockages through automated image analysis. As AI continues to evolve, it will play an increasingly vital role in refining how to diagnose heart disease with greater speed and accuracy. Conclusion The power of early, accurate diagnosis results in the following: Improved survival rates : Detecting disease at a treatable stage increases survival and remission rates for cancers and chronic illnesses. Reduced treatment intensity : Early intervention often requires less aggressive therapy, sparing men from extensive surgeries or high-dose, often toxic medications. Enhanced quality of life : Preventing complications maintains physical function, mental health, and independence. Cost savings : Early diagnosis reduces hospital stays, emergency care, and long-term management expenses. The integration of biomarkers into prostate cancer care is transforming diagnostic pathways from one-size-fits-all to highly personalized algorithms. Ongoing research aims to combine biomarker profiles with advanced imaging and artificial intelligence to detect cancer even earlier and more accurately. As these tools mature, men will benefit from fewer invasive procedures, smarter treatment choices, and better long-term outcomes. Early recognition of symptoms, a thorough understanding of risk factors, and the integration of traditional and innovative diagnostic tools are reshaping men’s health in both diagnosis and care. By leveraging advanced imaging, biomarker profiling, AI analytics, genetic insights, and wearable monitoring, physicians can detect disease earlier, initiate precise treatments, and significantly improve men’s health trajectories. Proactive screening and personalized diagnostics pave the way to longer, healthier lives. REFERENCES American Lung Association. Four diseases threatening men’s lung health. Lung.org. Accessed September 3, 2025. https://www.lung.org/blog/lung-diseases-threatening-men. Ellis RR. An overview of skin cancer. WebMD. December 31, 2006. Accessed September 3, 2025. https://www.webmd.com/melanoma-skin-cancer/skin-cancer. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150(1):17-22. doi:10.1001/jamasurg.2014.1756.

Featured in Medical Laboratory Observer - MLO Written By Lisa-Jean Clifford, President, Gestalt Planning is Essential As digital pathology and artificial intelligence (AI) continue to transform diagnostic medicine, laboratories find themselves at a timely and critical juncture. These technologies are not simply enhancements, they represent a fundamental shift in how clinical images and data are managed, routed, shared, viewed, interpreted and presented to pathologists. Laboratories that wish to remain at the forefront of innovation must evolve beyond traditional automation and digitally replicating their manual workflows. Instead, the lab who successfully implements these solutions must carefully evaluate its technical infrastructure to accommodate complex, image-centric workflows, multi-source data integration, and AI-assisted analysis. This evolution requires careful analysis and review of platforms that not only handle high-resolution whole slide images (WSIs), but also enable interoperable communication across diverse systems—such as scanners, LIS’s, EMR’s, EHR’s, and archives—using both native file formats and standardized protocols like DICOM. Successful implementation of digital pathology and AI depends upon a robust informatics ecosystem that supports interoperability and real-time data exchange between your applications. These include information that will be displayed in your viewer such as WSI’s, structured annotations, snapshots, AI overlays and results outputs but also includes patient specific case information like you traditionally see in your LIS. These tools must be securely embedded within workflows, ensuring compliance with privacy regulations, while remaining scalable for multi-site deployments. And, this transformation is not solely technical. It requires training and cultivating a digitally fluent workforce, with training initiatives that enable pathologists, lab (and scanning) technicians to navigate the components of the process seamlessly and accurately. Assess current infrastructure preparedness Before implementing new technologies, it’s essential for laboratories to assess their existing systems and infrastructure. This includes evaluating scanner compatibility, data storage capabilities, and network bandwidth to ensure readiness for high-volume imaging, robust case routing and AI integration. Understanding what's already compatible and supports this initiative can help identify gaps, avoid redundant investments, and lay the foundation for seamless upgrades. A thoughtful audit sets the stage for informed decisions and smoother transitions into digital pathology and automation. Key considerations include: Scanner selection – Do you already have scanners in place? If so, are the digital solutions you are evaluating compatible with all of them? Do the scanners fit the needs of your organization in terms of volumes, speed and quality of scanning, redundancy? Are they dated or current in terms of the technology? If they aren’t, or you don’t have scanners these are all items to consider in your evaluation and acquisition of scanners. Keep in mind that the better digital platforms will support multiple scanner brands and models, enabling you to mix and match the scanners that best suit your organizations needs. Information Management System(s) – Is your LIS/EMR/EHR interoperability ready or compatible? Is it an older, legacy system that will have limitations or challenges with being able to send and receive real-time data messages through standard methods of integration? Can your digital solution providers help bridge any of these gaps or do they have ways they can work around them in order to provide a seamless workflow for your pathologists and your technologists? If your information system or “source of truth” for the patient is current and easy to integrate with other solutions, do you have one or multiple you are looking to integrate with? Are you a multi-site entity or single location with one LIS or one version of that LIS? All of these are points to consider when determining: 1. Which digital solution you select; and 2. If there are other infrastructure changes you need to make to ensure that your applications are able to speak to each other effectively. Network Bandwidth – Most robust digital solutions today are web-based, as is the storage for these large images. It is essential to understand what your current network supports and to plan to make modifications prior to implementing a digital platform. Speed and performance are a huge factor in the successful usage and adoption of your platform. Most digital solutions are going to be optimized for performance, but internet and network bandwidth are two things that no vendor can account for. There are certainly things like load balancing and viewer performance that are important parts of the development and tuning process of vendors, however, providing the proper bandwidth and connectivity is something that the lab – or your IT department – will need to plan on. Storage options – Preplanning is an important step here as well. Knowing whether you are going to archive all of your images or some of your images and for what amount of time is a key consideration. Also understanding your options and selecting the one (or two) that are right for you is important. Are you planning to store locally/onsite? In the web? Does your digital solution provider offer both short – and long-term storage? Are you looking to use one of the large storage vendors (Google, AWS, Azure)? Understanding the price difference between these options, and if your digital solution provider can integrate bi-directionally with them is also a fundamental consideration. On this note – any decision you make in this category can be easily changed. Configure for Interoperability A successful digital pathology deployment depends upon integration, real-time data exchange and true interoperability. Support for image formats – Enables streamlined viewing, image sharing and best of breed selection for your organization across platforms. Are you looking to integrate your other ‘ologies’ and have a cross patient perspective by being able to collocate radiology and pathology images? If so, you also need to ensure that your systems support DICOM and integration with VNRs. Open APIs and HL7 – Enable seamless connectivity with LIS, AI algorithms, other lab applications and modules, and advanced analytics platforms Vendor-Neutral solutions – Avoid vendor lock-in by selecting digital platforms that offer interoperable viewers, databases, workflows and reporting. Choose AI vendors and algorithms that align with your needs AI algorithms should be carefully considered and evaluated to ensure that you address your specific clinical needs rather than adopt them as generic tools. Successful integration requires a thoughtful alignment by your digital pathology solution and the AI vendor. Those who have formed partnerships have put a great deal of thought and technical evaluation into the integration between the capabilities of their AI outputs and the digital workflow and visual representation to the pathologist. Whether it's assisting with tumor detection, streamlining case triage, or supporting specific diagnosis and cancer grading, AI solutions should be targeted to enhance clinical workflows and improve decision-making. By integrating AI deployment directly within your digital platform’s viewer, you are enabling your workflow to be grounded in the realities of patient care and operational demands, ensuring that laboratories can have a meaningful impact, increased efficiency, and better outcomes. Modernizing your lab’s infrastructure isn’t a single upgrade—it’s a strategic transformation. By focusing on compatibility, clinical utility, and scalable systems, labs can future-proof their operations and unlock the full potential of digital pathology and AI.
Blog

Interoperability is one of the most pressing challenges in digital pathology today—and one of the most critical to get right. In this webinar, Gestalt partnered with Barco for the fourth and final session of the year in a webinar series focused on advancing digital pathology workflows. The discussion centered on how seamless interoperability between scanners, viewers, and diagnostic display systems enables pathology teams to work more efficiently, collaborate with confidence, and plan for long-term digital transformation. Lisa-Jean Clifford and Hong Nguyen shared real-world perspectives on where interoperability breaks down, why open and flexible architectures matter, and how labs can modernize without increasing complexity for pathologists or IT teams. Who Should Watch Pathologists looking to support collaborative, digital workflows Lab managers focused on efficiency, scalability, and operational consistency IT specialists responsible for integration, security, and enterprise architecture Anyone involved in the digital transformation of pathology Date : December 9 Time : 11:00 AM ET Speakers : Lisa-Jean Clifford, President, Gestalt Hong Nguyen, Product Manager, Digital Pathology, Barko I f you missed the live session or would like to revisit the discussion, access the full webinar recording here .

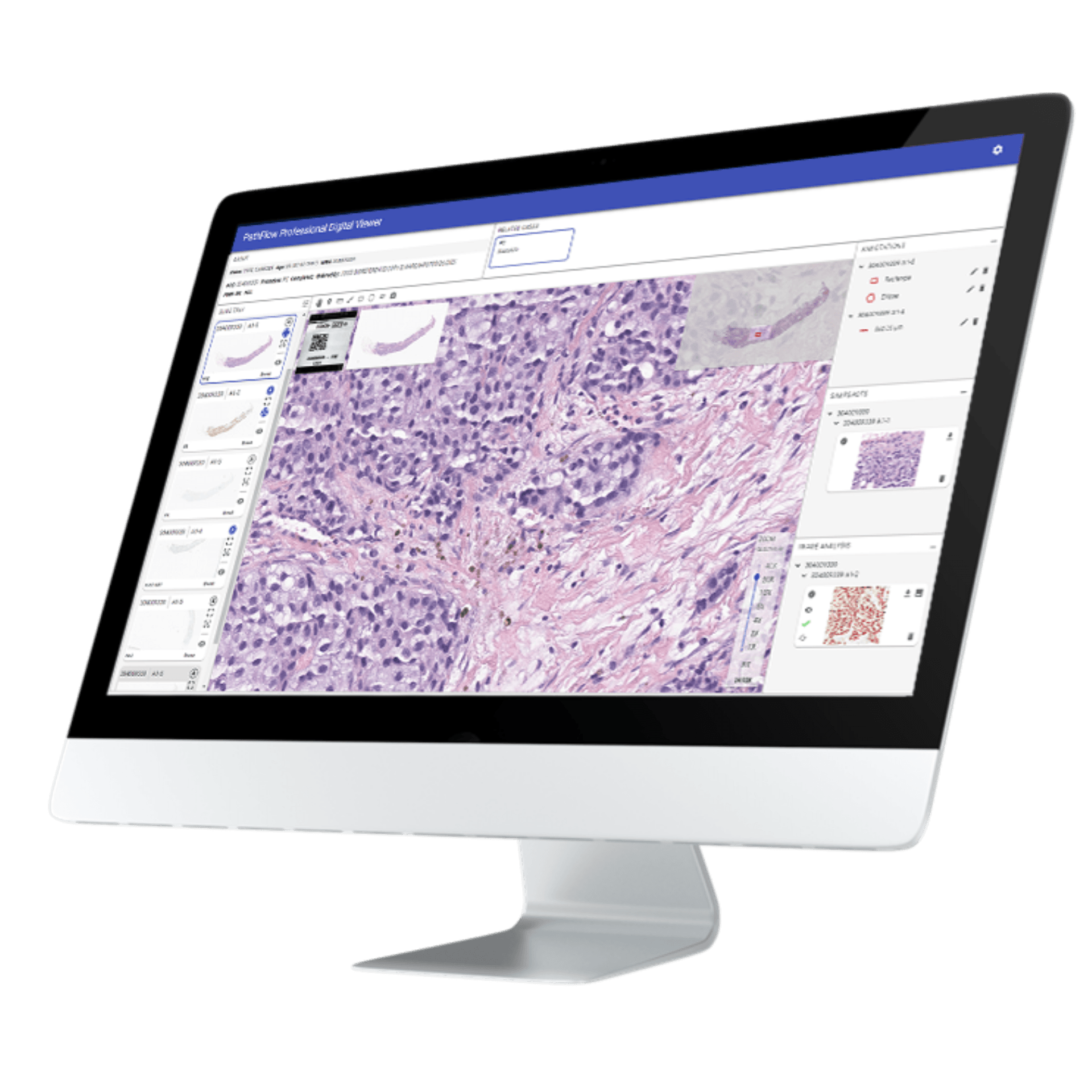
With the focus on digital pathology adoption around the globe today, efficiency and accuracy are an expected advantage for deployments in pathology labs of all sizes and types. With the continued advancement of these technologies, solutions like PathFlow and its lighter version, PathCloud, are revolutionizing the way pathologists manage their workflows. With PathFlow and PathCloud’s robust cloud-based capabilities, you have secure access your cases and images anywhere and anytime. With it truly interoperable infrastructure, PathFlow provides streamlined access, routing, and tools directly within the pathologists workflow. Pathology is becoming more in demand, case loads are increasing, pathologist availability is decreasing and the prediction for the number of new patients being diagnosed are all adding strain to the laboratory and the physicians. With a purpose designed infrastructure, PathFlow is made to decrease the manual workload and increase workflow optimization. PathCloud, the cloud-based lighter application to PathFlow, helps ensure pathologists can have access to tools and data from virtually anywhere on the globe in a cost effective, low footprint entry point to digital pathology. PathCloud’s secure environmen delivers real-time access to high-quality images and your case information. Both applications provide enhanced security and efficiency for your peace of mind.

By Maarion Napora At Gestalt, we understand the role of education for physicians and why it is important to have a focus specifically on pathology. It is our goal to provide the proper digital tools and platforms for pathologists to learn how to use the latest technology in their training. PathFlow ’ s Education Module provides academic medical centers and residency programs with leading tools and digital technology to train the next generation of pathologists. Designed to meet the demands of modern techniques, the module offers the ability to develop courses, easily clone and copy courses, and assign teaching lists, images, and tests. You can set the timing for a course, automatically send out messages to remind students of outstanding courses and test deadlines, etc. We support multiple forms of media, including videos, PowerPoint presentations, and word documents. These enable educators to deliver immersive, digital-first learning experiences. The PathFlow Education Module Revolutionizes pathology training by providing a unified platform for interactive learning. Trainees can access a comprehensive library of annotated digital slides, practice diagnosing complex cases, and receive real-time feedback. Professors can easily grade, provide comments and feedback on assessments, and can even assign certificates. The module’s credentialing management capabilities streamline competency assessments, notifications for deadlines, and reminders if not completed, ensuring residents meet rigorous standards while tracking progress seamlessly. We believe education and access to leading technology is the cornerstone of advancing pathology. With PathFlow’s Education Module, we’re empowering academic programs to train confident, tech-savvy pathologists, who will lead the field forward with current skills in available technology informatics.